Thanks @marko, I need to work through that and understand the error implications. Would it be straightforward for you to provide an example say at a propylene glycol mix of 20% and water temperature of around 35-40C?
Straightforward? No.
Checking is exercise for the reader I’m afraid as it’s not something that I have cause to do manually (you just buy meters programmed for it…) and finding the relevant data took more than 5 mins.
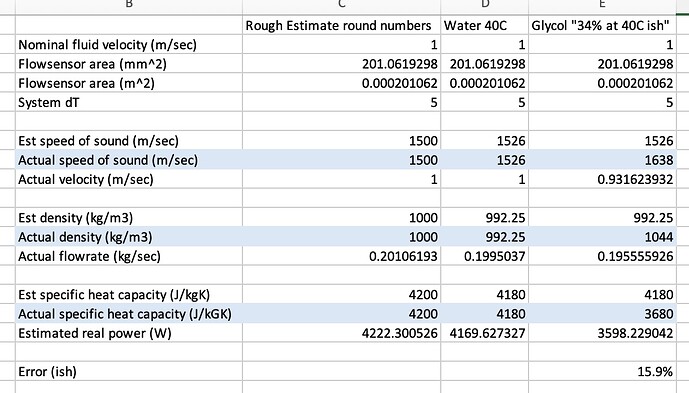
Pure water @ 40C / 1 bar
Speed of sound 1526 m/sec
Density 992.25 kg/m3
Specific heat capacity at constant pressure (Cp) 4.18 kJ/(kg*K)
Propylene Glycol I won’t do. That shouldn’t be used in heat pumps IMO because it’s S-T-I-C-K-Y as heck and you’ll increase your pumping costs materially.
Ethanol / alcohol is best and what we ought to be using (doesn’t freeze, isn’t sticky, and isn’t toxic) but the taxman kills that idea.
Ethylene glycol is therefore the common one. Doesn’t freeze. Not sticky. But toxic but ingestion for a while until it breaks down. (unless dosed to prevent uptake in animals somehow - detoxed ethylene glycol such as DTX)
Ethylene glycol @ X% and Y degC and Z bar?
Quite a bit more difficult to find data for!
This suggests 1638 +/- 3.5 m/sec over the range 15.6 degrees C-38.4 degrees C:
Or 1660 m/sec for pure ethylene glycol at 25C
The standard mixtures are 20% (-10C), 34% (-20c) and 52% (-40C)
At 34% you’re 1044 kg/m3 and 3.68 kJ/kgK at 40C
I think something like this BUT this was a quick hack so please check working…
Speed of sound is faster so less fluid volume has gone through than the sensor though. Density is higher though so this offsets the volume error to reduce the overall mass flowrate error. Then a larger error from the reduced specific heat capacity.
Glyocl.xlsx (9.9 KB)
Sanity check that though as it’s a 5 minute effort most of which was looking for glycol figures.
Thanks @marko much appreciated, looks like the Monoethylenglycol specific heat is contributing the largest component of the error in the calculation. Will spend some more time going through that for sure, thank you for your time outlining it so clearly. Interesting comments on Propylene, Ethanol and Ethylene. It would be interesting to understand how much the power consumption of the pump/s change with Propylene vs Ethylene at different mixtures and ultimate effect on COP.
I’ve moved this thread here so as to keep the original thread on topic.
For air source units…it’s probably moot. Unless you’re really in the sticks your most efficient / eco friendly option will be water and anti-freeze valves and dropping all the water out of the monobloc in the event of a power cut.
Flow in the heat exchangers is going to be turbulent regardless of what anti-freeze mixture, if any, is present. And they’re on the hot side. (glycol, like treacle, is runnier when hot)
Quickie calc from some Kiwis:
Of the order +30 to perhaps +50% pumping energy depending on the concentration used?
For ground source units…it very definitely needs to be considered at the design stage. The various books of words say* that flow in the ground array needs to not be laminar (barely into the transitional region is ideal) for decent heat transfer. That needs Reynolds number >2500 or so. Quite a high fluid velocity for plain pipe compared with a plate heat exchanger. You also need to deal with the fluid being cold (nominal operation around 0C, design condition perhaps -10C) and so very much stickier.
Scrambled notes follow:
Viscosity conversion
Ethylene glycol
Propylene glycol doesn’t have viscosity info here (I guess nobody pumps it - mostly used de-icing aircraft etc?)
Available here though:
At 40% concentration and 0F (sorry first chart to hand) you’re looking at 15 centipoise for ethylene glycol and 41 centipoise for propylene glycol.
MCS notes have handy charts:
Personal example:
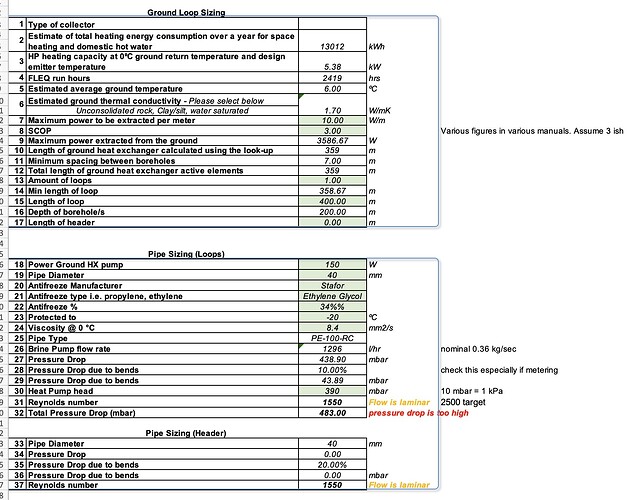
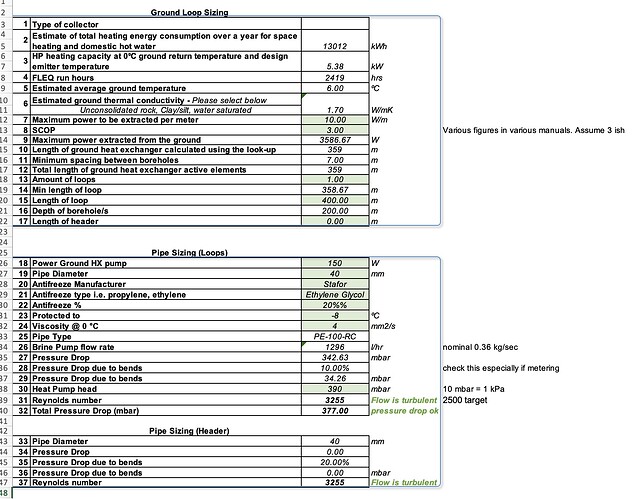
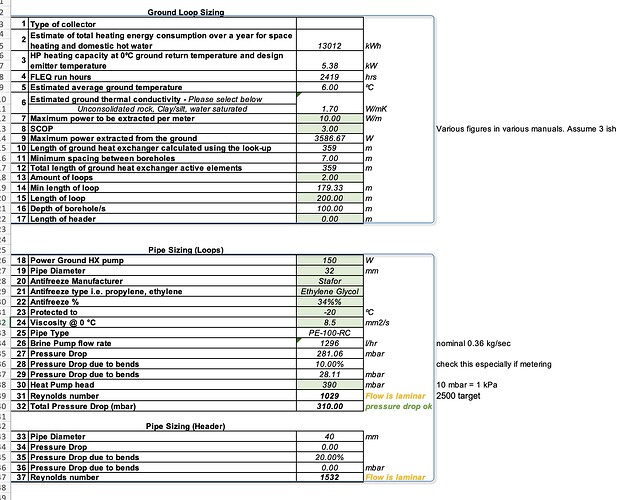
We are fiting a GSHP unit that asks for a -15C antifreeze mixture; and wants 0.36 kg/sec for ethylene glycol mixture. Available head is 39 kPa with the standard pump. (it’s designed for a runny alcohol-water mixture) It’ll be using a 400 metres of 40 mm HDPE in SDR17 dimension to suck heat from the ground.
Ethylene glycol tools:
https://engineeringnation.com/water-glycol/
I can’t actually make it work (non laminar flow AND within the available pump head) nicely with Ethylene Glycol due to the viscosity increase vs alcohol-water.
8.4 centistokes for a -20C rated mixture at -10C (safe and in accordance with manual)
4 centistokes for a -8C rated mixture at -5C (bit ballsy)
2x 200 metres by 32 mm instead of 1x 400 metres by 40 mm doesn’t work:
Not a bloody chance of that working with propylene glycol even if you were ballsy.
Pump energy for the circulators (ground loop and heating circuit) is 0.3 kW of the 1.32 kW total at brine 0C / water 45C - sizeable.
Choice here is to either replace the brine pump (with a higher head and more modern electronically commutated version) or buy 400 litres of vodka to fill that brine loop…
*I’m no pro on these as you can tell. We’re doing our first (DIY) install once it’s stopped snowing (and then melting) for long enough that the digger won’t sink in the peat. There’s a ruddy fine balance between turbulent flow and pump energy / available head / freezing points etc for ground loops. Air source far easier to not fudge up the design on but perhaps less suited to snow country.
Thanks @marko much appreciated, stunning location there! very jealous ![]()
Visitors that enjoy chopping logs or buying ground loops welcome any time! ![]()
This popped up as a promoted link on LinkedIn just now. Might be worth asking them to run some scenarios for you as an example of their software in action?